Super User
FAQs
Is Polyglycan a generic product?
No. Polyglycan® is a patented 3-component formula which has demonstrated characteristics beyond any of these components used individually. Polyglycan is specifically formulated to have the same viscosity and lubricating properties as natural equine synovial fluid.
Is Polyglycan considered a medical device or a drug?
Polyglycan is a medical device – it achieves therapeutic effect by mimicking the physical properties of equine synovial fluid. The mechanical lubrication and cushioning provided by Polyglycan helps by protecting the joint in a non-chemical/non-metabolic manner.
Is Polyglycan manufactured in an FDA-registered facility?
Yes! The Polyglycan manufacturing facility achieved Current Good Manufacturing Practice (CGMP) status over 10 years ago, and is routinely inspected by FDA.
Are products similar to Polyglycan ever used in humans?
Yes! In fact, all hyaluronic acid products currently used in humans for intra-articular joint treatments are also medical devices.
What is a medical device?
FDA determines a medical device as: "an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
- recognized in the official National Formulary, or the United States Pharmacopoeia, or any supplement to them,
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- intended to affect the structure or any function of the body of man or other animals which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes.”
What are the label claims for the uses of Polyglycan?
Polyglycan is specifically formulated to mimic the physical properties of normal equine synovial fluid. The product’s intended use is in the synovial space (directly in the joint) in horses as a post-surgical lavage, a synovial fluid replacement, or as a viscosupplement.
Robert Brusie
I use Polyglycan exclusively (other than biologics) in joint therapy. In other words, Polyglycan is my "go to" medication.
I have used Polyglycan since 2010. Since then our clinic has used over 70,000 doses in both post-surgical and treatment of typical inflammatory conditions of the joint. In my experience, I have very good to acceptable outcomes using Polyglycan in routine joint therapy. Additionally, we have not experienced the occasional joint flair seen in other products. We have not had any problems with any type of proliferative arthritic conditions. Currently, I use Polyglycan exclusively (other than biologics) in joint therapy. In other words, Polyglycan is my "go to" medication.

Robert W. Brusie, DVM, Diplomat ACVS
Palm Beach Equine Clinic, Wellington, FL
Research
Equine Studies
Safety when used in horses:
Fred J. Caldwell, DVM, MS, DACVS, Earl M. Gaughan, DVM, DACVS, Melissa Fagerlin, BS BS Dept. of Clinical Sciences, College of Veterinary Medicine, Auburn University, Auburn, Alabama 36830
Safety Evaluation of Polyglycan® in Horses
Safety in horses when used at 1 & 3 times normal dose:
Safety Study on the Effects of Intra-articular Polyglycan at 1X and 3X Doses
CE Kawcak DVM, PhD, Diplomate ACVS Associate Professor, Equine Orthopaedic Research Laboratory, Colorado State University 10-14 08
Efficacy In horses with IA use:
Frisbie DD; Kawcak CE; McIlwraith CW; Werpy NM
Report on the Evaluation of Intra-Articular Polyglycan® for Osteoarthritis using an Equine Model
36th Annual Orthopedic Society Meeting, February 28 - March 6, 2009, Steamboat Springs, Colorado
Compared to other joint treatments:
David D. Frisbie, DVM, PhD, Diplomate ACVS, & ACVSMR Equine Orthopaedic Research Center, Department of Clinical Sciences, College of Veterinary Medicine and Biological Sciences, and Molecular, Cellular & Tissue Engineering, Colorado State University, Fort Collins, Colorado, 80523
Current Evidence on Treatments of Joint Disease
2nd Equine Lameness Research Workshop, Oklahoma City, OK July 24, 2012
Compared to controls in equine joints:
Christopher E. Kawcak, DVM, PhD, Dipl ACVS, C. Wayne McIlwraith, BVSc, PhD, DSc, FRCVS, Dipl ACVS, Gail Holmes Equine Orthopaedic Research Laboratory, Colorado State University, Fort Collins, CO
Comparison of Synovial Fluid in Middle Carpal Joints Undergoing Needle Aspiration, Infusion with Saline, and Infusion with a Combination of N-Acetyl-D-Glucosamine, Hyaluronic Acid, and Sodium Chondroitin Sulfate
Efficacy against single impact load:
Frances M. D., Henson, MA, Vet MB, PhD; Alan M. J. Getgood, MD; David M. Caborn, MD; C. Wayne McIlwraith, DVM, PhD, DSc; Neil Rushton,
Effect of a solution of hyaluronic acid–chondroitin sulfate–N-acetyl glucosamine on the repair response of cartilage to single-impact load damage
American Journal of Veterinary Research, February 2012, Vol. 73, No. 2, Pages 306-312 doi: 10.2460/ajvr.73.2.306
Companion Animal Studies
Safety in dogs, given IA or IM:
MacPhail CM, Brewer M, Hawley JR, Vier J, Lappin MR.
Local & systemic effect of a commercial preparation of CS, HA and NACD-glucosamine when administered IA or IM to healthy dogs
Proceedings of the American College of Veterinary Internal Medicine Annual Forum (abstract), New Orleans, LA June 1, 2012 (poster)
Get in Touch
Questions?
Call us at 1-888-524-6332 or
Email us at This email address is being protected from spambots. You need JavaScript enabled to view it.
Bimeda US Office
Polyglycan

Over a million doses administered by trusted veterinarians since 2006
Canine Use
Canine Polyglycan Products Comparison
Patented 3-component formula

Polyglycan-SA
Single Administration - aka Small Animal
Other Non-Polyglycan Injectable Joint Products
Canine Administration
Instill Polyglycan® into the synovial space to replenish lost synovial fluid. Quantity to be determined by Veterinarian Surgeon. Discard any unused portion of vial contents.
Dr. Paul Shealy, DVM, MS, DACVS demonstrates intra-articular injection technique for the major joints in companion animals. This educational, instructional video is provided as a guide for those practitioners interested in understanding proper injection technique. ArthroDynamic's Polyglycan-SA, is a sterile post-surgical joint lavage, synovial fluid replacement & viscosupplement product and is shown in use throughout this video.
Equine Use
Equine Polyglycan Products Comparison
Patented 3-component formula

Polyglycan
Not available yet in China

Polyglycan-HV
High Viscosity
Not available yet in China

Polyglycan-SA
Single Administration
aka Small Animal
Other Non-Polyglycan Injectable Joint Products
Equine Administration
Instill Polyglycan® into the synovial space to replenish lost synovial fluid. Quantity to be determined by Veterinarian Surgeon. Discard any unused portion of vial contents.
About Polyglycan and Joints
Polyglycan® is a patented formulation designed to replace lost or damaged synovial fluid. It contains naturally-occurring components of the synovia that play a central role in maintaining the homeostatic environment of the joint.
Properties
- Glycosaminoglycans are important components of all extracellular tissue structures including cartilage and synovial fluid.
- The active components in Polyglycan® show viscoelastic and polyionic properties similar in nature to synovial fluid.
- Patented 3-component formula has demonstrated characteristics beyond any of these components used individually.
Benefits 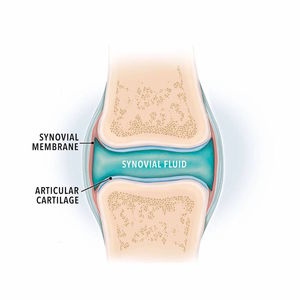
- Help replenish lost synovial fluid with naturally occurring elements
- Assist in normalization of synovial fluid viscosity
- May be used for post-surgical lavage of the joint cavity and surfaces
C. Wayne McIlwraith
"In the treatment efficacy portion, joints of Polyglycan® treated horses had fewer microscopic articular cartilage abnormalities as well.
There has apparently been no report of any clinical or radiographic consequences in this location with clinical use in the horse.”
C. Wayne McIlwraith, BVSc, PhD, DACVS, DACVSMR
Orthopaedic Research Center, CSU
David D. Frisbie, PhD, DACVS, DACVSMR
Orthopaedic Research Center, CSU
Excerpts from a summary document prepared by the Co-Principal Investigators of a CSU study in response to concerns raised from their paper that the use of intravenous administration of Polyglycan® might cause harm.
C.R. Johnson
Polyglycan is administered intra-articularly and intravenously in my pracitce. I administer Polyglycan intra-articularly during and after joint surgery, and the horses are administered Polyglycan weekly for, at least, 4 weeks after surgery
"Polyglycan (hyaluron, sodium chondroitin, N-acetyl-D-glucosamine) is administered in my practice. I administer Polyglycan during and after joint surgery (arthroscopy or arthrotomy), and the horses are administered Polyglycan weekly for, at least, 4 weeks after surgery, if financially feasible. A large part of my practice is arthroscopic removal of OCD lesions and chip fractures in Thoroughbred yearlings. The vast majority of these joints do not have radiographic or clinical signs of osteoarthritis prior to surgery. I routinely perform post-operative films within 30 days after surgery, and a large number of these horses have radiographs taken for use at auction several months after surgery. I have not observed osteophyte formation after administering Polyglycan in these cases for the past 9 years.
In my opinion, there is decreased joint morbidity in the cases where Polyglycan is administered after surgery versus the cases that do not receive Polyglycan. I also observe decreased joint morbidity and quicker resolution of effusion in the race horses and yearlings that have clinical and radiographic evidence of osteoarthritis prior to surgery."
C.R. Johnson, DVM, MS, DACVS
Equine Surgical Services, Versailles, KY

Chris Morrow
Polyglycan® is the primary product for joint injections in my equine practice since 2006.
Polyglycan® has been administered extensively to horses competing in a variety of disciplines. This population includes professional rodeo horses, cutting horses, reining horses, working cow horses, racehorses, and horses in the English disciplines ... We’ve used over 50,000 mL of Polyglycan® in our practice giving us a high level of comfort and confidence in its applications. To date there have been no adverse events to be reported by our clients or our practice. Through my peer group I have not had conversations to suggest that their experience has been different.

Chris Morrow, DVM
Mobile Veterinary Practice, Amarillo, TX

Robert J. Hunt
I have continued to use Polyglycan as a standard postoperative parenteral treatment as well for essentially all of my orthopedics.
"I began using Polyglycan in a clinical setting over 10 years ago, initially as a post-surgical lavage following arthroscopic surgery. Soon afterward I began routine administration on a weekly or bi-weekly basis on athletes with musculoskeletal maintenance issues as well as young developing horses with developmental orthopedic disease. I have continued to use Polyglycan as a standard postoperative treatment as well for essentially all of my orthopedics. I have encountered no adverse effects from either route of administration of Polyglycan.
In addition to using Polyglycan in racing athletes and sport horses, I routinely recommend its use in weanlings and yearlings with synovitis and joint effusions that are the result of osteochondrosis lesions. I also frequently administer Polyglycan in foals undergoing treatment for septic arthritis. I have not experienced any adverse reactions with the use of Polyglycan."
Robert J. Hunt, DVM, MS, Diplomate, ACVS
Hagyard Equine Medical Institute, Lexington, KY